İlker Büyükyavuz 1, Saniye Ekinci 1, Mithat Haliloğlu 2, M. Emin Şenocak 1, Arbay O. Çiftçi 1 Departments of 1 Pediatric Surgery, and 2 Radiology, Hacettepe University Faculty of Medicine, Ankara, Turkey
SUMMARY: Büyükyavuz İ, Ekinci S, Haliloğlu M, Şenocak ME, Çiftçi AÖ. A case of patent ductus venosus with pulmonary arterio-venous fistula as a rare and unique clinical entity. Turk J Pediatr 2004; 46: 272-274.
A 13-year-old boy presenting with digital and lip cyanosis, easy fatigability, and weight gain was diagnosed to have an intrapulmonary arterio-venous fistula. During his routine follow-up examinations, there was fullness on right upper quadrant of his abdomen. Abdominal ultrasonography (USG) showed a mass in portal hilus. We planned abdominal computed (CT) to gain tomography detailed information about the mass. Surprisingly abdominal CT and the color-Doppler sonography showed that the structure mimicking a mass was patent ductus venosus (PDV).
We report PDV with intrapulmonary arterio-venous fistula as a unique and rare clinical entity. In addition, we underline the misdiagnosis of portal mass instead of patent ductus venosus. If there is a portal mass in USG or CT, color- Doppler sonography or, if needed, other diagnostic methods should be used to exclude PDV.
Key words: portosystemic shunting, patent ductus venosus, pulmonary arterio-venous fistula, hepatic dysfunction, hypoxemia.
Patent ductus venosus (PDV) is a very rare clinical entity in children1. PDV may cause liver dysfunction and eventually encephalopathy. Rarely pulmonary arterio-venous fistulas may accompany, and this may cause hypoxemia and other cardiac symptoms. If enzymatic defect cannot be found in hypergalactosemia, we should consider the diagnosis of PDV, in other words a portosystemic shunting.
Here in we report a case of PDV with a review of the literature and the rare, special conditions related to PDV.
Case Report
A 13-year-old boy was admitted to the pediatric cardiology department with the complaint of digital and lip cyanosis, weakness, and no weight gain. In the patient’s history, he had a swelling on the left knee and had an operation for this mass. The histopathology of the mass was vascular malformation with multiple capillaries and striated muscle tissue.
On physical examination, there was 2° systolic murmur. Physical examination revealed right upper abdominal quadrant fullness and digital
clubbing. We did not note any familial disease in the history of the patient. There were no specific features in prenatal and natal history.
His laboratory findings did not show any abnormalities: hemoglobin, 15.9 g/dl; leukocyte count, 4400/mm3; and thrombocyte count, 118000/mm3, normal. Liver enzymes and serum bilirubin levels were within normal ranges. Arterial blood gas showed some decrease in oxygen saturation and pulse of oxygen (pulse of oxygen, 60.2; saturation of oxygen, 89.3), but the pH of blood and pulse of carbon dioxide were normal. Posteior-anterior X-ray of the thorax showed the suspected bronchiectatic area in the right lung. Thorax computed tomography (CT) showed bilateral centrilobular opacities and minimal peribronchial thickening. Because of the symptom of cyanosis, electrocardiography (ECG), echocardiography (ECHO) and angiography were planned for the patient. ECG was normal, but ECHO and angiography showed pulmonary arterio-venous fistula on the right lung with mitral valve prolapsus. Cranial magnetic resonance imaging (MRI) was made to exclude any intracranial
arterio-venous malformation, and revealed bilateral symmetric hyperintense accumulation of the paramagnetic substance. However, it did not show arterio-venous fistula or any vascular malformation. Meanwhile he had an operation for the recurrent mass at his left knee. The histopathology of the mass was hemangioma.
Because of the right upper quadrant fullness of the abdomen, he had an abdominal ultrasonography (USG), which showed a mass (60x34x50 mm) at the portal hilus and chronic parenchymal changes in the liver. Liver biopsy revealed scattered hepatocellular necrosis. To obtain more information about the mass, abdominal CT was planned, which revealed hypertrophy of the caudate lobe of the liver and a shunt between the portal vein and inferior vena cava. Color-Doppler was then made, showing intrahepatic porto-caval shunt (patent ductus venosus), chronic parenchymal liver disease and splenomegaly (Fig. 1). Because the cardiac symptoms of the patient were tolerable and he did not have liver dysfunction despite Doppler sonographic findings, he was not operated for either patent ductus venosus pulmonary arterio-venous fistula. The patient is under close follow-up. He is doing well now except for recurrent masses of the left knee.
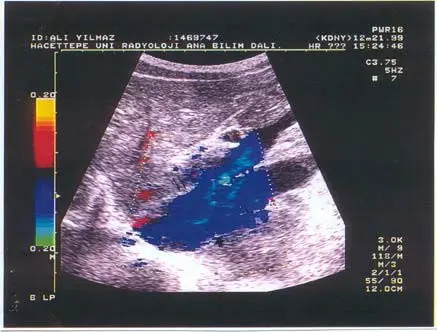
Fig. 1. Longitudinal color-Doppler ultrasonography demonstrates the large communication (thin arrows) between the portal vein and inferior vena cava (thick arrow).
Discussion
During fetal life, ductus venosus is the continuation of the umbilical vein. It originates from the left portal vein and ends in the region of the hepatic vein at the point of confluence with the inferior vena cava. There are different results
reported concerning its closure time. Some authors believe it closes functionally in the first few minutes but closure may not be completed until 15 to 20 days after birth. However, others think that the ductus closure is not seen until two to four days after birth. In one study, complete functional closure of the ductus venosus occurred at two weeks of age in 93% of infants, and this was followed by anatomic closure. In premature infants, ductus venosus may stay open more than 15-20 days2-4.
There are different opinions regarding pathophysiology of the closure of ductus venosus: Since ductus venosus reopens in portal hypertension, any abnormalities of portal and hepatic venous system, i.e. the absence of the intrahepatic portion of the inferior vena cava, may cause patency of ductus venosus. PDV with atrophic right lobe of the liver and hypoplasia of the right portal vein without portal hypertension has been reported. Umbilical venous catheters may also cause the PDV by way of portal thrombosis. All the above- mentioned causes lead to a vascular resistance, and this keeps the ductus venosus open5-7.
There are limited reports of PDV in the pediatric age group1,6-10. Patent ductus venosus, or congenital portosystemic shunting, is a very rare clinical entity and may cause portosystemic encephalopathy. As in our case, PDV with intrapulmonary shunting is an exceedingly rare clinical entity and may cause hypoxemia sometimes with cyanosis and digital clubbing in the absence of encephalopathy and hepatic dysfunction8,11. In the literature, PDV with coronary artery fistulas has also been reported10. As a diagnostic method, I-iodoamphetamine per-rectal portal scintigraphy is a useful and noninvasive technique for portosystemic shunt12. In laboratory findings, hyperammonemia and elevated bile acid level are important for diagnosis and to follow the progress of disease7. Angiography, Doppler ultrasonography and CT are other diagnostic procedures. Sometimes in CT and ultrasonography, PDV might give mass images, which may be misdiagnosed as a mass like in our case2,13.
Patent ductus venous is a very rare clinical entity alone and PDV with pulmonary shunting, as in our case, is a unique and extremely rare case. In differential diagnosis of portal masses, we suggest color-flow Doppler ultrasonography because PDV may be misdiagnosed as a portal mass with gray-scale ultrasonography.
REFERENCES
- Bellah RD, Hayek J, Teele RL. Anomalous portal venous connection to the suprahepatic vena cava: sonographic demonstration. Pediatr Radiol 1989; 20: 115-117.
- Loberant N, Barak M, Gaitini D, Herskovits M, Ben-Elisha M, Roguin N. Closure of the ductus venosus in neonates: findings on real-time gray-scale, color-flow Doppler, and Doppler sonography. AJR 1992; 159: 1083-1085.
- Mitchell IM, Pollock JC, Gibson AA. Patient ductus venosus. Pediatr Cardiol 1991; 12: 181-183.
- Loberant N, Herskovits M, Barak M, et al. Closure of the ductus venosus in premature infants: findings on real-time gray-scale, color-flow Doppler, and duplex Doppler sonography. AJR 1999; 172: 227-229.
- Meyer WW, Lind J. The ductus venosus and the mechanism of its closure. Arch Dis Child 1966; 41: 597-605.
- Maisawa S, Takasago Y, Oyake Y, Maeta H, Fujiwara T. Patent ductus venosus with hypoplastic right hepatoportal system in a young child born with asymmetric intra-uterine growth retardation. Eur J Pediatr 1992; 151: 569-572.
- Uchino T, Endo F, Ikeda S, Shiraki K, Sera Y, Matsuda I. Three brothers with progressive hepatic dysfunction and severe hepatic stetosis due to a patent ductus venosus. Gastroenterology 1996; 110: 1964-1968.
- Kamata S, Kitayama Y, Usui N, et al. Patent ductus venosus with a hypoplastic intrahepatic portal system presenting intrapulmonary shunt: a case treated with banding of the ductus venosus. J Pediatr Surg 2000; 35: 655-657.
- Gitzelmann R, Arbenz UV, Willi UV. Hypergalactosaemia and portosystemic encephalopathy due to persistence of ductus venosus arantii. Eur J Pediatr 1992; 151: 564-568.
- Mori K, Dohi T, Yamamoto H, Kamada M. An enormous shunt between the portal and hepatic veins associated with multiple coronary artery fistulas. Pediatr Radiol 1990; 21: 66-68.
- Orii T, Ohkohchi N, Kato H, et al. Liver transplantation for severe hypoxemia caused by patent ductus venosus. J Pediatr Surg 1997; 32: 1795-1797.
- Daniel GB, Bright R, Ollis P, Shull R. Per rectal portal scintigraphy using technetium99m pertechnetate to diagnose portosystemic shunts in dogs and cats. J Vet Intern Med 1991; 5: 23-27.
- Ohnishi K, Hatano H, Nakayama T, Kohno K, Okuda K. An unusual portal systemic shunt, most likely through a patent ductus venosus: a case report. Gastroenterology 1983; 85: 962-965.
